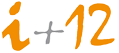On 13 January 2016, the new Royal Decree 1090/2015 came into effect, regulating clinical trials with drugs, the Research Ethics Committees with medicines and the Spanish Registry of Clinical Studies. This royal decree seeks to adapt Spanish legislation to make viable the current and future application of Regulation (EU) No. 536/2014 and to develop those aspects that the regulation leaves to national legislation, including the Ethic Committee for Research with medicinal products. Several aspects are introduced that involve a novelty with respect to the Committees, starting with the denomination itself as the Research Ethics Committees with medicinal products (ECR). In addition, it is worth highlighting the recognition of the integration of the ECR in the development of the Law 14/2007, of 3 July on biomedical research.
All the documentation referring to this new legislation is posted on the website of the Spanish Agency for Medicines and Medical Devices.